Hospira (Pfizer)
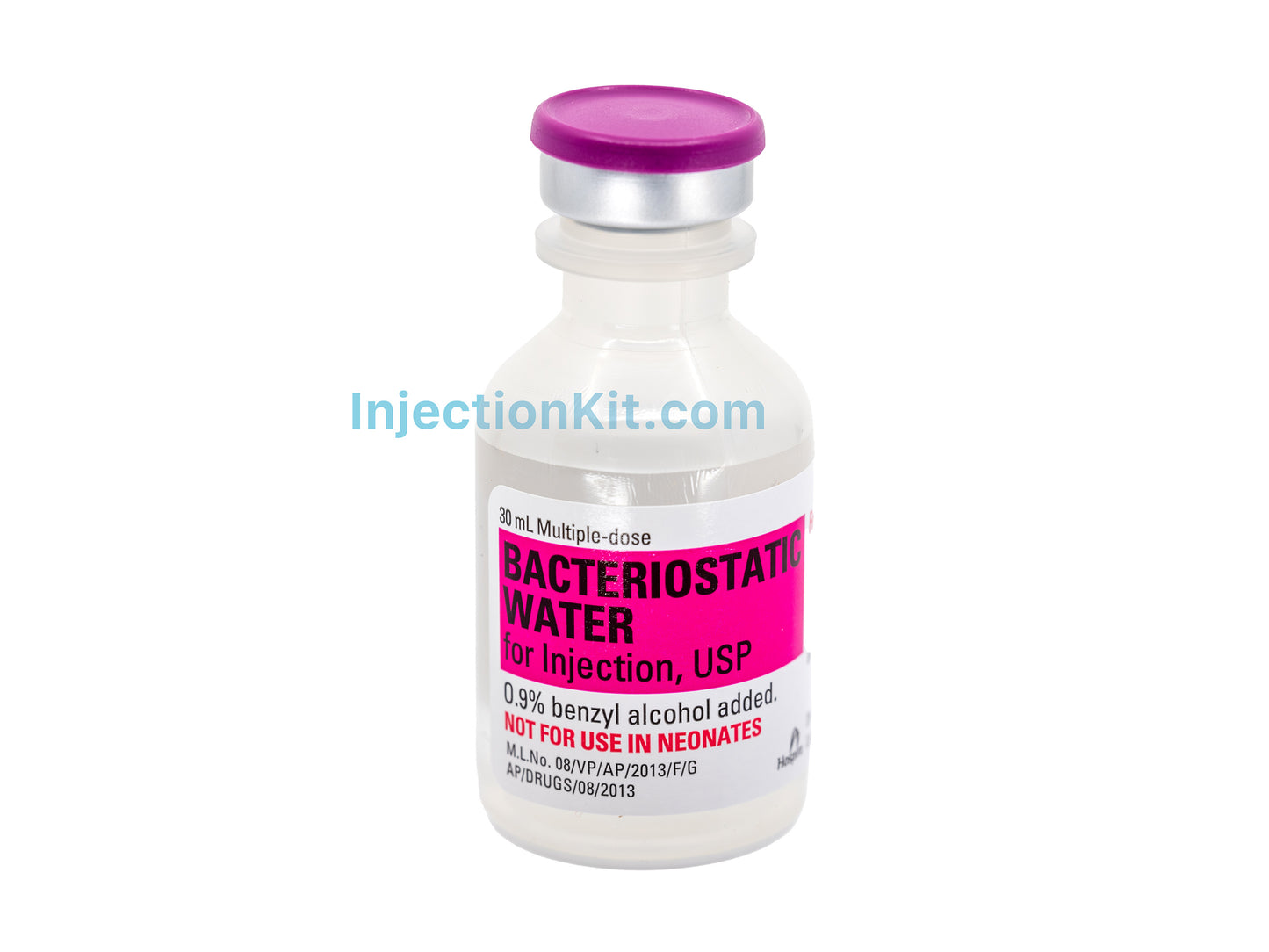
Hospira Bacteriostatic Water
Hospira Bacteriostatic Water
Couldn't load pickup availability
Brand: Hospira (Pfizer)
Dispensed in a 30mL, plastic, multi-dose vial. Product is FDA Approved.
Designed for administration via injection following the addition of medications that require dilution or must be dissolved or reconstituted with water before injecting.
Bacteriostatic Water for Injection with benzyl alcohol, USP is: chemically designated H2O and is a sterile, non-pyrogenic preparation of water containing 0.9% (9mg/mL) of benzyl alcohol added as a preservative and has a pH of 5.7.
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed according to USP biological standards for plastic containers. It requires no vapor barrier to maintain the properly labeled volume.
(Please note you may receive either of the two bottle label designs (Pfizer or Hospira) - these are identical products from the same manufacturer. The only difference is the design of the label, based on whether it was originally made for the Canadian or US market. We carry both and consider them to be interchangeable.)
Share